EU GMP Certification
Active Pharmaceutical Ingredient at Biosyyd.
Certified.
A certificate of Good Manufacturing Practice (GMP) is issued to a manufacturer by the national competent authority that carried out an inspection if the outcome of the inspection confirms that the manufacturer complies with the principles of Good Manufacturing Practice, as provided by European Union legislation.
At Biosyyd we can proudly state that we have been successfully audited by the STATE MEDICINES CONTROL AGENCY OF LITHUANIA.
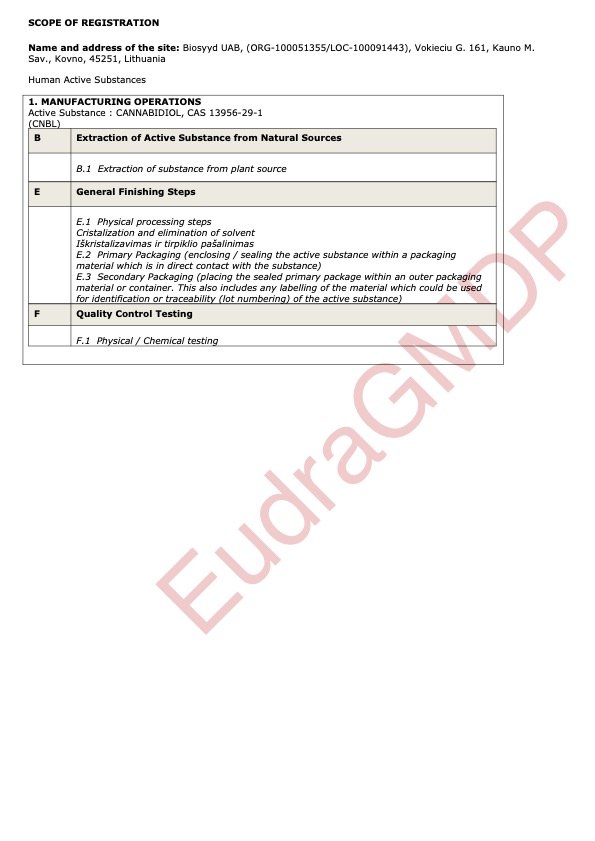
Our natural Cannabidiol (CBD) Isolate complies with the current EUROPEAN PHARMACOPOEIA 11.5 and the Monograph 07/2024:3151 for CANNABIDIOL (CAS 13956-29-1).
Our certificate can be verified under EudraGMDP with:
Registration Number: VM-0016
EudraGMDP Document Reference Number: 48131
OMS Organisation Identifier: ORG-100051355
OMS Location Identifier: LOC-100091443
NCA Site Reference Number: 1717064169232
EudraGMDP Site Reference Number: 214927
Good Manufacturing Practice. What is it?
Any manufacturer of medicines intended for the EU market must comply with GMP.
Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production. The main risks are:
contamination of products
incorrect labelling
insufficient or excessive active ingredient
GMP ensures that every step of production follows strict, documented protocol. The areas of inspection include:
materials
premises
equipment
training
personal hygiene of staff
GMP requires that medicines or medical ingredients:
are of consistently high quality
are appropriate for their intended use
meet the requirements of the marketing or clinical trial authorisation
Documented systems ensure that correct procedures are consistently followed at each step in the manufacturing process — every time a product is made.